
|
|
PRINT » |
|
|
E-MAIL THIS PAGE » |
|
|
CLOSE THIS WINDOW » |
Grape-Derived Fruity Volatile Thiols
Adjusting Sauvignon Blanc aroma and flavor complexity

Sulfur-containing compounds in wine include a wide range of typically very powerful aromatic molecules. These compounds are referred to as mercaptans or volatile thiols, both indicating compounds containing the sulfhydryl group (-SH). Wine industry and research circles have grown accustomed to some of these generic terms being used for specific compounds.
For example, the term "volatile thiols" (sometimes called "varietal thiols") immediately sparks a preconceived reference to fruity and tropical aromas, while "mercaptans" generally refer to compounds contributing to the reductive odors reminiscent of cabbage and burnt match. In some cases, the term "sulfur" is used to describe either of these odor groups or even refer to the typical "chemical" smell when sulfur dioxide is picked up sensorially and could cause confusion during evaluations.
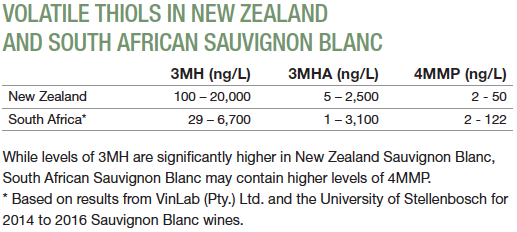
Volatile thiols in wine are important compounds contributing to the varietal character of wine made from several cultivars, notably Sauvignon Blanc. These sulfur-containing compounds are one of the main reasons for the success observed in Sauvignon Blanc produced in Marlborough, New Zealand, with concentrations soaring to levels only imagined in most wine-producing countries.
They are not limited to one grape variety, and significant contributions also have been observed in Chenin Blanc, Riesling, Colombard, Semillon, Cabernet Sauvignon and Merlot.
However, Sauvignon Blanc has enjoyed the most attention when it comes to these potent molecules, due to the fact that large quantities can be found in wines produced from this cultivar.
Research conducted at Stellenbosch University in South Africa involved screening South African Chenin Blanc wines to investigate the impact of volatile thiols on the sensory characteristics of this variety. Preliminary results look promising and could affect how this variety is handled in the vineyard and cellar. Research of volatile thiols has been a hot topic in the past 10 years. Studies conducted at various universities around the globe have only recently clarified formation and stability of the aromatic compounds.
One reason for this could be difficulty surrounding the analysis of volatile thiols. Volatile thiols are present in wine at ng/L (parts per trillion), which complicates the quantification significantly.
Various methods using a sophisticated gas chromatograph mass spectrometer in a commercial or university laboratory (with lengthy and tedious sample preparation) are employed to successfully and accurately measure volatile thiols in wine. Trace concentrations present in wine have the ability to generate various fruity descriptors due to the very low olfactory detection threshold (lowest concentration at which 50% of the population are able to detect the aroma).
Types of volatile thiols and their sensory thresholds
Three main volatile thiols are responsible for the tropical fruit nuances in wines. They are 3MH (3-mercaptohexan-1-ol), 3MHA (3-mercaptohexyl acetate) and 4MMP (4-mercapto-4-methylpentan-2-one). The smell is quite potent (or "punchy," as the Kiwis say) at higher concentrations, and descriptors used include tropical fruit, passionfruit, grapefruit, guava, gooseberry, box tree, tomato leaf and black currant. Perception thresholds for 4MMP, 3MH and 3MHA in model wine are 0.8 ng/L, 60 ng/L and
4.2 ng/L, respectively.
Recently it was discovered that volatile thiols contribute to the "green" odors in wine such as green pepper and stalky attributes generally assumed contributed only by methoxypyrazines (another aroma group significantly adding to Sauvignon Blanc flavor).1,2 Recently wine sensory evaluations have seen the use of the term "thiols" on the tasting sheets (unpublished data). The term has not been properly defined, however it is assumed to refer to the general group of attributes that is brought by the volatile thiols. The fact that the volatile thiols can contribute to both fruity flavors and green odors can be challenging.
The concentration of these impact compounds in wine will influence the intensity of the attributes, however the concentration may also determine the descriptor used to describe the wine.1,3 For example, "lower" concentrations of 3MHA will impart passion fruit, guava and gooseberry odors while a sweaty aroma develops at higher concentrations.
Cat pee (box tree) as an aroma descriptor has been used at elevated volatile thiol (4MMP) concentrations. However, during aging, the concentration of the thiols will change due to degradation (such as the hydrolysis of 3MHA to 3MH) or oxidation, and with this alteration a change in aroma attributes may occur. When this happens, the wines that contained an initial higher concentration of volatile thiols might display fruitier aromas for a longer period of time compared to the wines that contained lower initial quantities.
Concentrations of volatile thiols found in international wines vary significantly, with Marlborough wines generally taking first place.
The effect of "minor" differences in thiol concentrations between wines is under review. In wines where 3MH concentration varied from 0 ng/L to 875 ng/L, the aroma of the wines did not greatly differ,2 and it seems a higher quantity of thiols are needed to really have an impact on the wine aroma (even though concentrations greatly exceed perception threshold).
It might be worth investigating at what specific concentration the volatile thiols will start having an influence on specific white wines from South Africa. The effect of the wine matrix and the presence of other aromatic and non-aromatic compounds on the perception of the volatile thiols should be considered. Suppressing and enhancing effects greatly influence how these compounds are perceived.
Origin of volatile thiols
The volatile thiols (4MMP and 3MH) are not present in grapes and are only released/formed from precursors to their volatile and pleasant-smelling form during alcoholi
c fermentation. 3MHA (an acetate ester) is formed from 3MH through yeast-driven acetylation and does not normally exceed the concentration of 3MH in the final wine.4
 During the 1990s and 2000s, research regarding participation of the cysteine (Cys-3MH) and glutathione (Glut-3MH) bound precursors came to the fore.5 A substantial amount of work has been done to understand these precursors. In follow-up fermentations, it has been conclusively proven that these conjugated precursors are not the main precursors and only contribute to a fraction of the formed volatile thiols.6 Recently, other similar precursors such as Glut-3MH-Al and Glut-3MH-SO3 have been identified,7 but these still only account for a small percentage of volatile thiols formed during fermentation. Other conjugated compounds are being investigated as potential precursors.
During the 1990s and 2000s, research regarding participation of the cysteine (Cys-3MH) and glutathione (Glut-3MH) bound precursors came to the fore.5 A substantial amount of work has been done to understand these precursors. In follow-up fermentations, it has been conclusively proven that these conjugated precursors are not the main precursors and only contribute to a fraction of the formed volatile thiols.6 Recently, other similar precursors such as Glut-3MH-Al and Glut-3MH-SO3 have been identified,7 but these still only account for a small percentage of volatile thiols formed during fermentation. Other conjugated compounds are being investigated as potential precursors.
The conjugated precursors are not released linearly, and a low (0.1%-12%) conversion rate when using commercial wine yeast was observed, as well as an almost complete lack of correlation between the bound precursor concentrations and the final thiol concentrations in wine.8 Even with this information widely known and accepted, these conjugated precursors are still commonly referred to as being the main source of volatile thiols in wine, though the statement remains unsupported. The presence of these precursors does, however, excite the scientific community. The fact that the conversion is so low suggests a lot of potential in the juice. A method to draw this through to the wine would be key.
Other pathways for the formation of volatile thiols are being investigated that may be more significant sources than conjugated precursors. These pathways include the contribution of H2S (or another -SH moiety) as a sulfur donor in combination with a C6 compound such as (E)-2-hexenal or (E)-2-hexenol, during the first few hours of fermentation.9 This is a more direct pathway for the formation of 3MH. However, this reaction is not a straightforward chemical reaction and requires the presence of yeast activity (possibly enzymatic), as tests have shown that the reaction does not take place in un-inoculated media.
These observations imply that the formation of 3MH and /or 3MHA via the H2S pathway requires yeast activity and suggest that it is not driven by straightforward chemical reactions.
C6 compounds such as hexenal and hexenol are formed from C18 polyunsaturated fatty acids in plant cell membranes. This reaction occurs rapidly during the damaging of plant cells due to the oxidation of the polyunsaturated fatty acids in the presence of oxygen and lipoxygenase enzymes. The grape damage occurs during harvesting and processing.
Why does the plant/berry produce C6 compounds in "stress" situations? Evolutionarily speaking, the reaction makes great sense: The C6 compounds have anti-microbial properties, and this reaction (which occurs in berry-damaging situations) will lead to production of toxic-protecting compounds (in this case the C6 compounds) to minimize the potential damage caused by the specific injury or infection. As a result, the (somewhat damaged) grapes have elevated concentrations of these protecting C6 compounds. What is known is that (E)-2-hexenal has a proven inhibitory and fungicidal activity against Saccharomyces cerevisiae, 10 which is probably one of the reasons that yeast metabolizes it, together with its related alcohol (E)-2-hexen-1-ol, into the less toxic and less reactive hexan-1-ol.
The tripeptide glutathione already naturally present in the grape tissue can react with C6 compounds to yield the non-toxic Glut-3MH precursor, in this way protecting the berry from elevated levels of C6 compounds. A thiol precursor is formed during the detoxification process!
In a similar manner, it is hypothesized that yeast inoculated into the grape must will disable the toxic compounds in its own way by converting, for example, hexenol to hexanol and by facilitating the binding of antioxidants with these C6 compounds, thereby resulting in precursor/volatile thiol formation. The plant and/or yeast will use mechanisms to convert the toxic substances formed during berry damage to an inactive form resulting in an odorous product. H2S has been considered to possibly be a main antioxidant binding compound for the binding of these C6 compounds. However, production of H2S by the yeast does not coincide with the uptake of these C6 compounds (within 24 hours after inoculation during the yeast lag phase) and other sources can be considered.
This suggests the availability of another sulfur donor in the early fermentation. However, again a huge untapped potential remains in that large amounts of C6 compounds are present during early fermentation stages that can be converted to volatile thiols if the correct sulfur donor is available. A patent is currently pending to allow the bubbling of H2S through the grape must during early stages of fermentation to facilitate the reaction of C6 compounds with H2S. This addition is currently illegal except for research wines produced using this process.
In a study where H2S was added to the must before fermentation, an unprecedented amount of 257 µg/L of 3MH and 35 µg/L 3MHA were present in the finished wines. These concentrations are up to 38 times the amount found in "high thiol" wines. Hexenol and hexenal formation is not unique to Sauvignon Blanc-or other "thiol-containing cultivars," for that matter-they can occur in musts from all grape varieties.
If the bubbling of H2S is approved, the application should be done with great caution due to the unpleasant sensory effect unreacted H2S can have on wine aroma and the toxicity of H2S. Using a more natural approach by encouraging the earlier formation of H2S by yeast or delaying the disappearance of C6 compounds could have the same effect. The extent to which this occurs in commercial winemaking needs further study.
The presence of elemental sulfur can have an effect on the thiol concentration in wines. Where increased amounts of elemental sulfur were added to Sauvignon Blanc grape must, an increase in the volatile thiols was also observed. However, these increases were accompanied with increases in reductive compounds such as H2S and methyl-thiol and corresponding reductive
aroma attributes.9
These processes seem promising, however the exact origin of the volatile thiols in wine continues to elude the scientific community at present. The race to expose the pathway involved in volatile thiol formation is a constant pressure point in the research community due to the implications it will have on production of all wine varieties, especially Sauvignon Blanc. The secret to this mechanism will unlock huge potential and a wealth of possibilities. Other than controlling thiol production in the winery, it will provide viticulturists and winemakers with the tools needed to predict the thiol potential of grapes in vineyards. Until then, the procedures known to increase volatile thiol levels in wine should be exploited in order to ensure maximum thiol production if that is what the winemaker is going for.
Dr. Carien Coetzee completed her Ph.D. at the University of Stellenbosch. Her studies evolved around the effect of oxidation on Sauvignon Blanc wines with a central theme of aromatic compounds and their stability. She is currently employed at Vinlab, an accredited laboratory supporting the South African wine industry. Contact her at carien@vinlab.com.
References
1. Coetzee, C., J. Brand, G. Emerton, D. Jacobson, A.C. Silva Ferreira and W.J. du Toit. 2015 "Sensory interaction between 3-mercaptohexan-1-ol, 3-isobutyl-2-methoxypyrazine and oxidation-related compounds." Aust. J. Grape Wine Res. 21 (2), 179-188.
2. Kilmartin, P. 2016 "Thiols found in Sauvignon Blanc and their significance." In New Zealand Society for Viticulture & Oenology Sauvignon Blanc Workshop; 24-80.
3. van Wyngaard, E., J. Brand, D. Jacobson and W.J.; du Toit. 2014 "Sensory interaction between 3-mercaptohexan-1-ol and 2-isobutyl-3-methoxypyrazine in dearomatised Sauvignon Blanc wine." Aust. J. Grape Wine Res. 20 (2), 178-185.
4. Swiegers, J.H., R. Willmott, A. Hill-Ling, D.L. Capone, K.H. Pardon, G.M. Elsey, K.S. Howell, M.A. de Barros Lopes, M.A. Sefton, and M. Lilly. et al. 2006 "Modulation of volatile thiol and ester aromas by modified wine yeast." Dev. Food Sci. 43 (C), 113-116.
5. Coetzee, C. and W.J. du Toit. 2012 "A comprehensive review on Sauvignon Blanc aroma with a focus on certain positive volatile thiols. Food Res. Int. 45 (1), 287-298.
6. Subileau, M., R. Schneider, J.M. Salmon and E. Degryse. 2008 "New insights on 3-mercaptohexanol (3MH) biogenesis in Sauvignon Blanc wines: Cys-3MH and (E)-hexen-2-al are not the major precursors." J. Agric. Food Chem. 56 (19), 9230-9235.
7. Thibon, C., C. Böcker, S. Shinkaruk, V. Moine, P. Darriet and D. Dubourdieu. 2016 "Identification of S-3-(hexanal)-glutathione and its bisulfite adduct in grape juice from Vitis vinifera L. cv. Sauvignon Blanc as new potential precursors of 3SH." Food Chem. 199, 711-719.
8. Pinu, F.R., S. Jouanneau, L. Nicolau, R.C. Gardner and S.G. Villas-Boas. 2012 "Concentrations of the Volatile Thiol 3-Mercaptohexanol in Sauvignon Blanc Wines: No Correlation with Juice Precursors." Am. J. Enol. Vitic. 63 (3), 407-412.
9. Harsch, M.J., F. Benkwitz, A, Frost, B. Colonna-Ceccaldi, R.C. Gardner and J.M. Salmon. 2013 "New precursor of 3-mercaptohexan-1-ol in grape juice: Thiol-forming potential and kinetics during early stages of must fermentation." J. Agric. Food Chem. 61 (15), 3703-3713.
10. Kubo, I., K.I. Fujita, A. Kubo, K.I. Nihei and C.S. Lunde. 2003 "Modes of antifungal action of (2E)-alkenals against Saccharomyces cerevisiae." J. Agric. Food Chem. 51, 3951−3957.
11. Jouanneau, S. 2011 "Survey of aroma compounds in Marlborough Sauvignon Blanc wines. Regionality and small scale winemaking," University of Auckland.
12. Coetzee, C. and W.J. Du Toit. 2015 "Sauvignon Blanc wine: Contribution of aging and oxygen on aromatic and non-aromatic compounds and sensory composition - a review." South African J. Enol. Vitic.
13. Kritzingera, E.C., M.A. Standerb and W.J. Du Toita. 2013 "Assessment of glutathione levels in model solution and grape ferments supplemented with glutathione-enriched inactive dry yeast preparations using a novel UPLC-MS/MS method." Food Additives & Contaminants: Part A, Vol. 30, No. 1, 80-92,
14. Rosales del Prado, D. 2015 "Effect of glutathione and inactive yeast additions to Sauvignon Blanc at harvest on wine aroma." University of Auckland.
15. Herbst-Johnstone, M., l. Nicolau and P.A. Kilmartin. 2011 "Stability of varietal thiols in commercial sauvignon Blanc wines. Am. J. Enol. Vitic. 62 (4), 495-502.
16. Olejar, K.J., B. Fedrizzi and P.A. Kilmartin. 2015 "Influence of harvesting technique and maceration process on aroma and phenolic attributes of Sauvignon Blanc wine." Food Chem. 183, 181-189.
17. Maggu, M., R. Winz, P.A. Kilmartin, M.C.T. Trought and L. Nicolau. 2007 " Effect of Skin Contact and Pressure on the Composition of Sauvignon Blanc Must." J. Agric. Food Chem. 55 (25), 10281-10288.
18. Parish, K.J. M. Herbst-Johnstone, F. Bouda, S. Klaere and B. Fedrizzi. 2016 "Pre-fermentation fining effects on the aroma chemistry of Marlborough Sauvignon Blanc press fractions." Food Chem. 208, 326-335.
19. Allen, T., M. Herbst-Johnstone, M. Girault, P. Butler, G. Logan, S. Jouanneau, L. Nicolau and P.A. Kilmartin. 2011 "Influence of grape-harvesting steps on varietal th ol aromas in sauvignon Blanc wines." J. Agric. Food Chem. 59 (19), 10641-10650.
20. Coetzee, C., K. Lisjak, L. Nicolau, P. Kilmartin and W.J. du Toit. 2013 "Oxygen and sulfur dioxide additions to Sauvignon Blanc must: Effect on must and wine composition." Flavour Fragr. J. 28 (3), 155-167.
21. Moine, V., M.L. Murat, C. Arfeuillère and C. Thibon. 2011 "Collage des jus de presse Blanc. Influence sur leurs teneurs en composes phénoliques, en glutathion et précurseur d'arômes." Rev. des œnologues 138, 45-47.
22. Lee, S.A., f.E. Rick, J. Dobson, M. Reeves, H. Clark, M. Thomson and R.C. Gardner. 2008 "Grape juice is the major influence on volatile thiol aromas in Sauvignon Blanc." Aust. N.Z. Grapegrower & Winemaker 533 (6), 78-86.
23. Anfang, N., M. Brajkovich and M.R. Goddard. 2009 "Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in sauvignon Blanc." Aust. J. Grape Wine Res. 15 (1), 1-8.
24. Masneuf-Pomarède, I., C. Mansour, M. Murat, T. Tominaga and D. Dubourdieu. 2006 "Influence of fermentation
te5perature on volatile thiols concentrations in Sauvignon Blanc wines." Int. J. Food Microbiol. 108 (3), 385-390.
23. Makhotkina, O., B. Pineau and P.A. Kilmartin. 2012 "Effect of storage temperature on the chemical composition and sensory profile of Sauvignon Blanc wines." Aust. J. Grape Wine Res. 18 (1), 91-99.
26. Coetzee, C., J. Brand, D. Jacobson and W.J. du Toit. 2016 "Sensory effect of acetaldehyde on the perception of 3-mercaptohexan-1-ol and 3-isobutyl-2-methoxypyrazine." Aust. J. Grape Wine Res.
27. Parish, K. 2012 "The significance of pressing conditions on key aroma volatiles in Marlborough Sauvginon Blanc," University of Auckland.
|
|
PRINT » |
|
|
E-MAIL THIS ARTICLE » |
|
|
CLOSE THIS WINDOW » |
